Study #1
- Science Inside: Rao, Amanda, et al. “Influence of a specialized Trigonella foenum‐graecum seed extract (libifem), on testosterone, estradiol and sexual function in healthy menstruating women, a randomised placebo controlled study.” Phytotherapy Research 29.8 (2015): 1123-1130.
- Study Design: Randomized, double-blind, placebo-controlled trial
- Subjects: 80 menstruating women aged 20-49 who reported low sex drive
- Intervention: 600mg / day
- Study Duration: 8 weeks

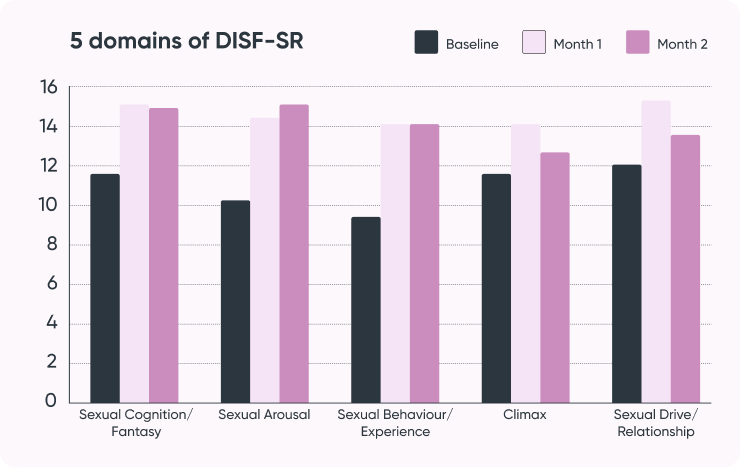
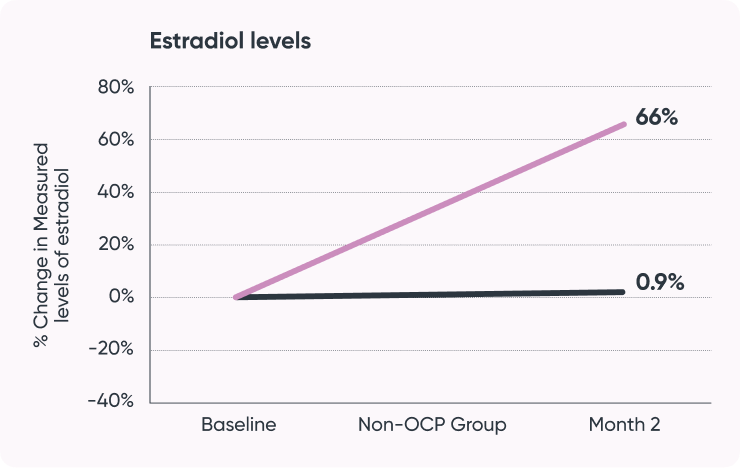
Study result:
- Increased estradiol
- Increased sexual desire and arousal
Study #2
- Science Inside: Steels, E., Steele, M.L., Harold, M. and Coulson, S., 2017. Efficacy of a proprietary Trigonella foenum‐graecum L. de‐husked seed extract in reducing menopausal symptoms in otherwise healthy women: a double‐blind, randomized, placebo‐controlled study. Phytotherapy Research, 31(9), pp.1316-1322.
- Study Design: Randomized, double-blind, placebo-controlled trial
- Subjects: 115 women aged 40-65
- Intervention: 600mg / day
- Study Duration: 12 weeks

Efficacy of Trigonella foenum-graecum de-husked seed extract supplemented group and placebo group on patient-reported
(over 7 days) number of vasomotor episodes at T 0 , T 4 , T 8 and T 12 weeks.
Legend: Active treatment is 600 mg/day of Trigonella foenum-graecum de-husked seed extract.
A) Day-time flushes;
B) Night sweats;
C) Total day-time flushes and night sweats (over 7 days).
Active group (dark pink), placebo group (light pink).
Significance at p < 0.05, GEE, auto-regression covariance, Poisson distribution used, 5% error bars.
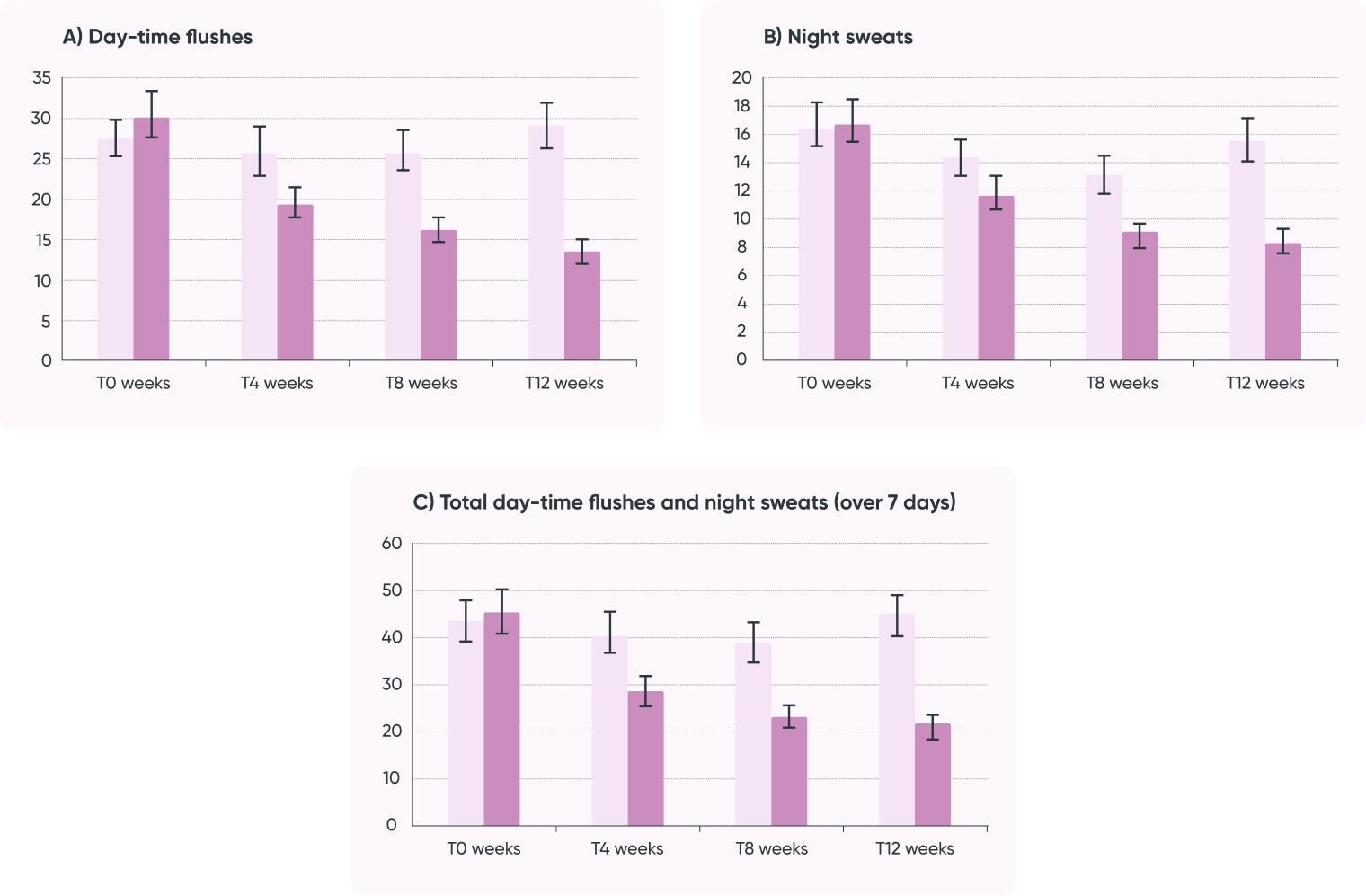
Study result:
- Reduced menopausal symptoms (hot flashes & night sweats)
- Improvement in vasometer, psychosocial, physical, and sexual symtpom domains
Study #3
- Science Inside: Libifem® (Trigonella foenum-graecum) in conjunction with exercise on muscle strength, power, endurance, and body composition in females aged between 25 and 45 years
- Study Design: Randomized, double-blind, two-active, one placebo-controlled trial
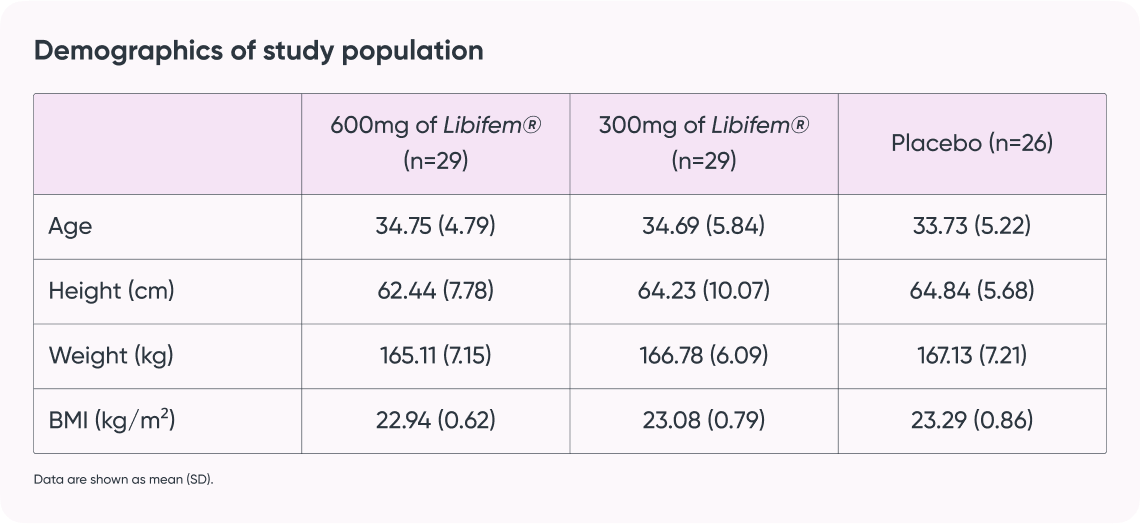
- Subjects: 129 women aged 25-45
- Intervention: 300mg/day or 600mg/day
- Study Duration: 8 weeks

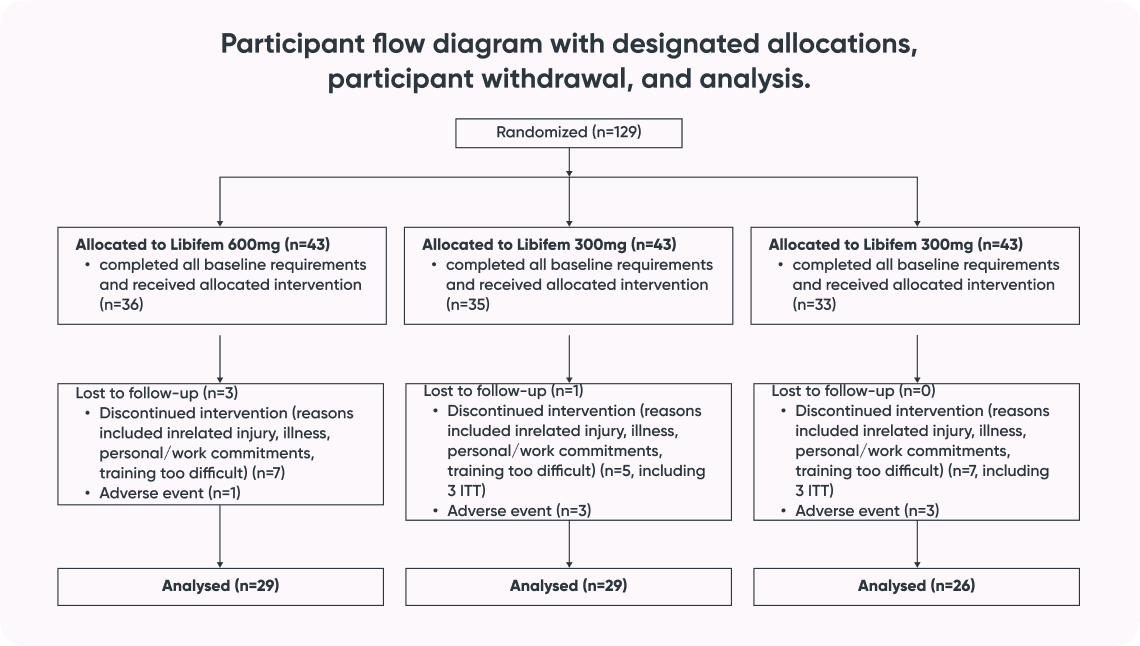
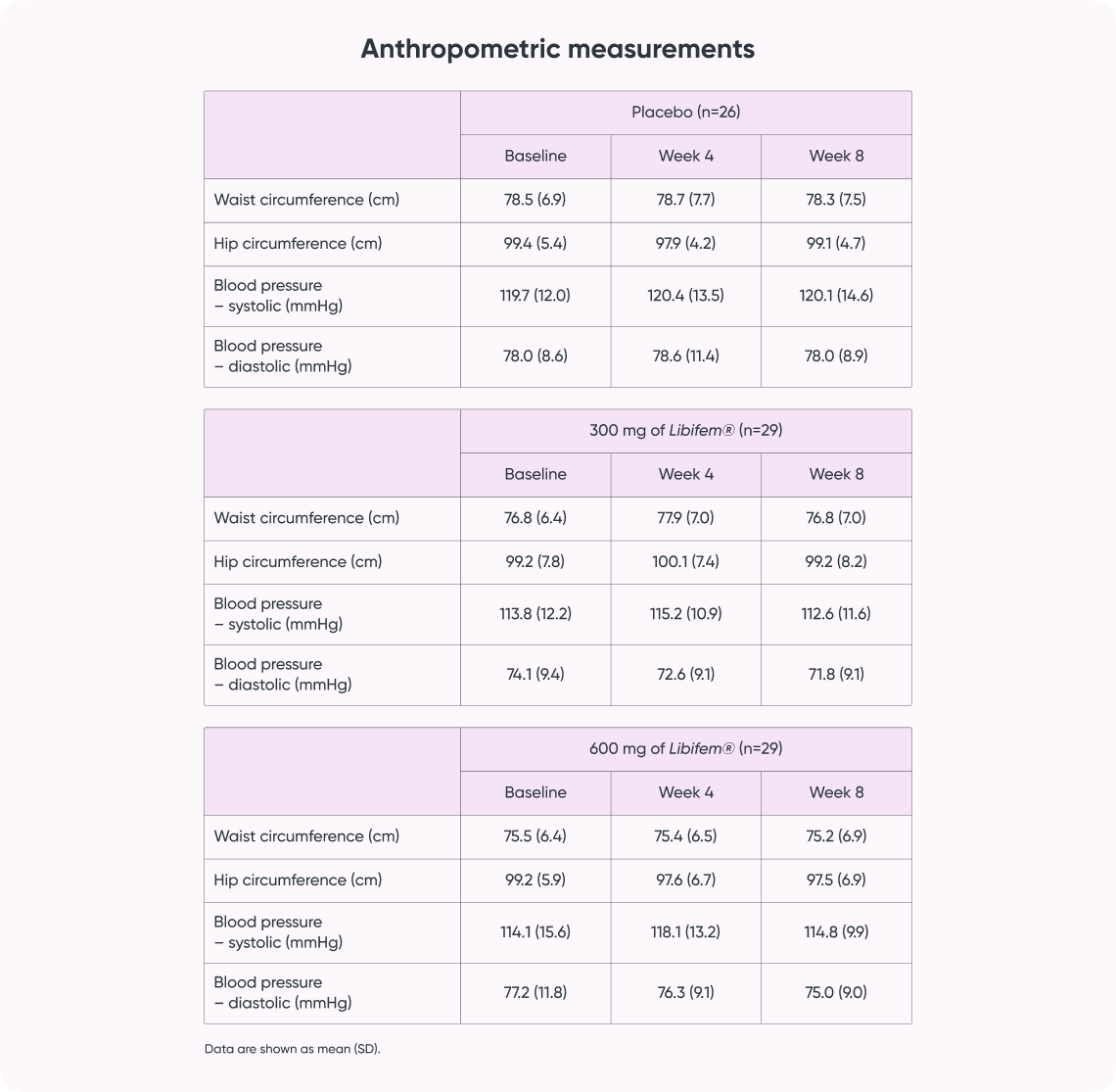
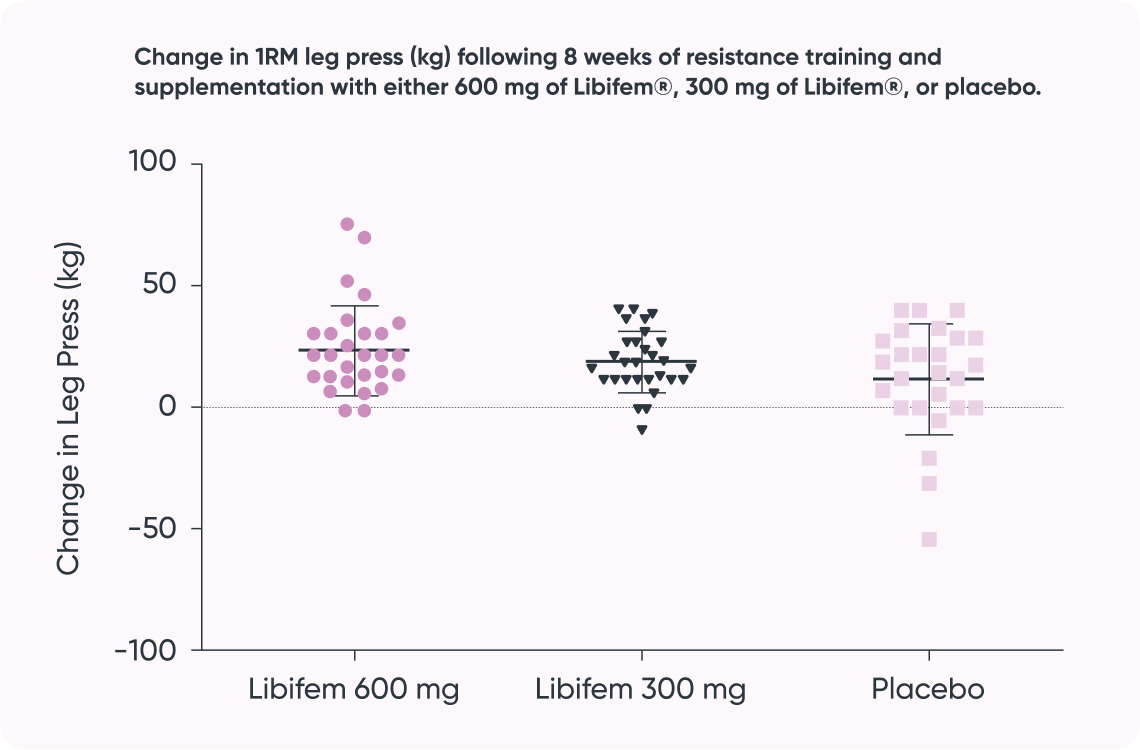
A significant difference of between-group treatment effect was seen for leg press at week 8 [F (2, 82) = 0.122, p = 0.045]. All three groups improved their 1RM leg press from baseline to week 8 (22.17 kg, 17.68 kg, and 10.12 kg for the 600 mg, 300 mg, and placebo groups, respectively). The 600 mg Libifem® group significantly improved (p = 0.014) from baseline compared with the placebo at week 8.
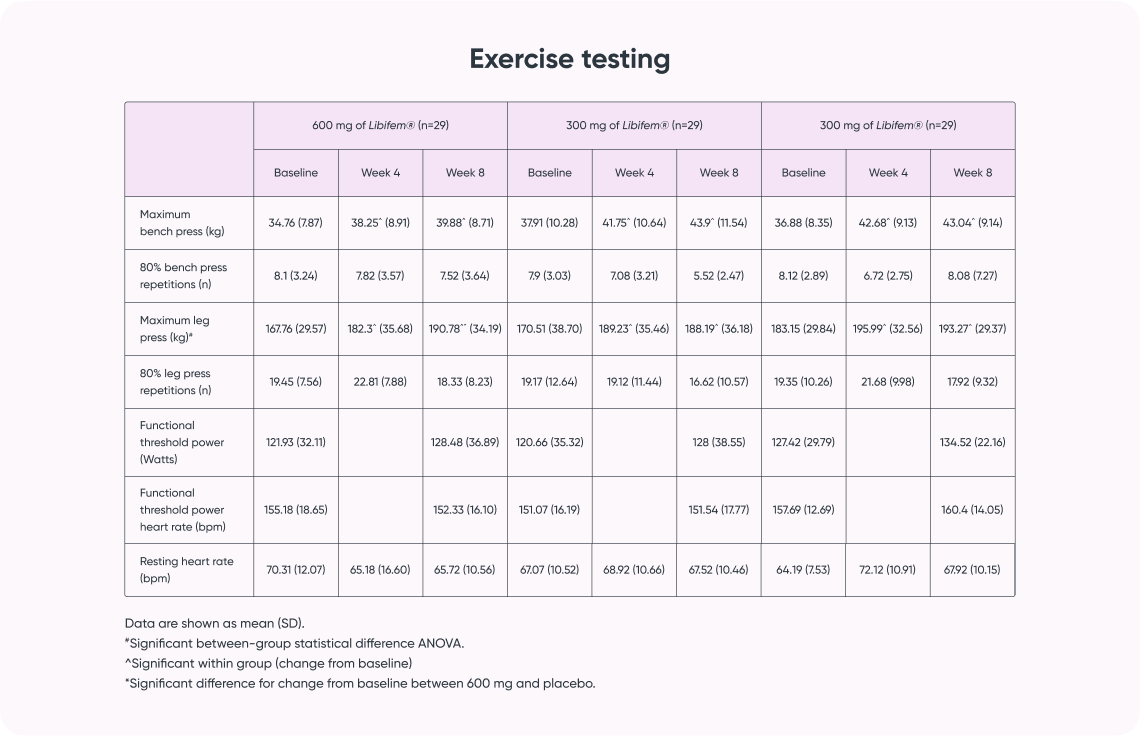
Significant increases (p < 0.05) from baseline in 1RM bench press were observed for all three groups (5.12 kg 600 mg Libifem®, 5.98 kg 300 mg Libifem®, 6.15 kg placebo); however, no significant differences between the groups were found. No differences were observed over time or between groups for the 80% max bench or leg press repetitions.
Significant increases (p < 0.05) from baseline in 1RM bench press were observed for all three groups (5.12 kg 600 mg Libifem®, 5.98 kg 300 mg Libifem®, 6.15 kg placebo); however, no significant differences between the groups were found. No differences were observed over time or between groups for the 80% max bench or leg press repetitions.
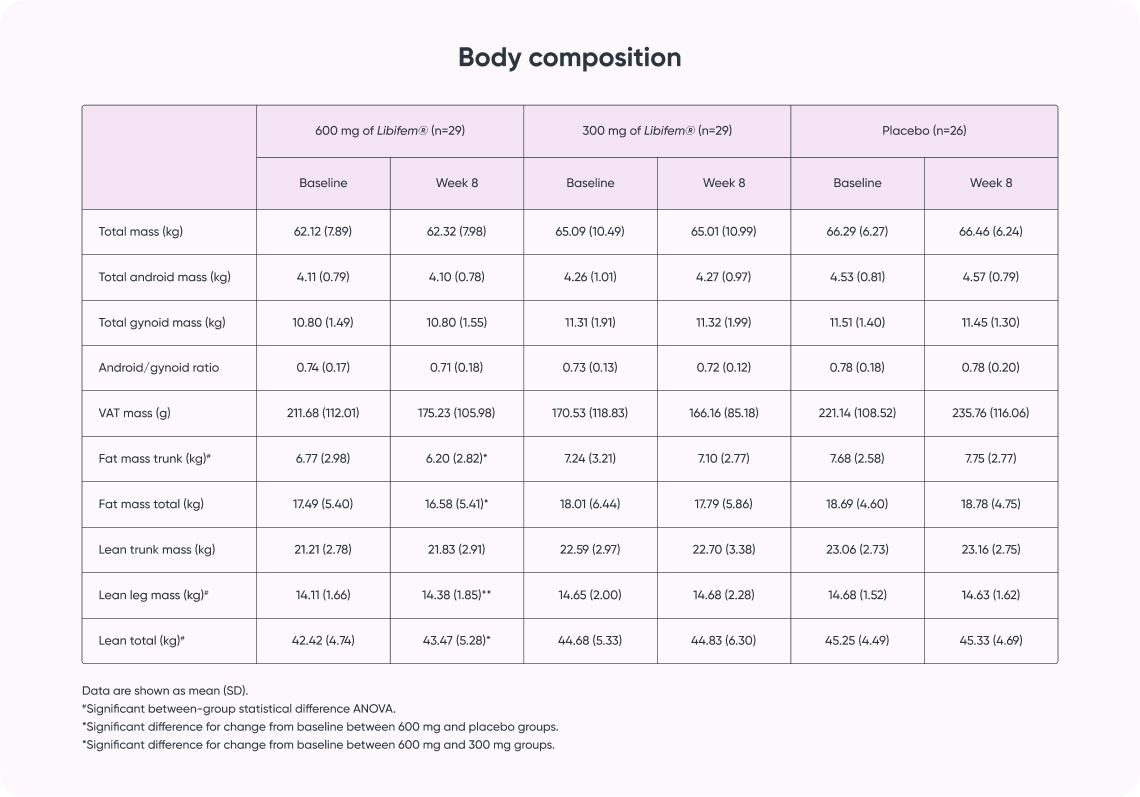
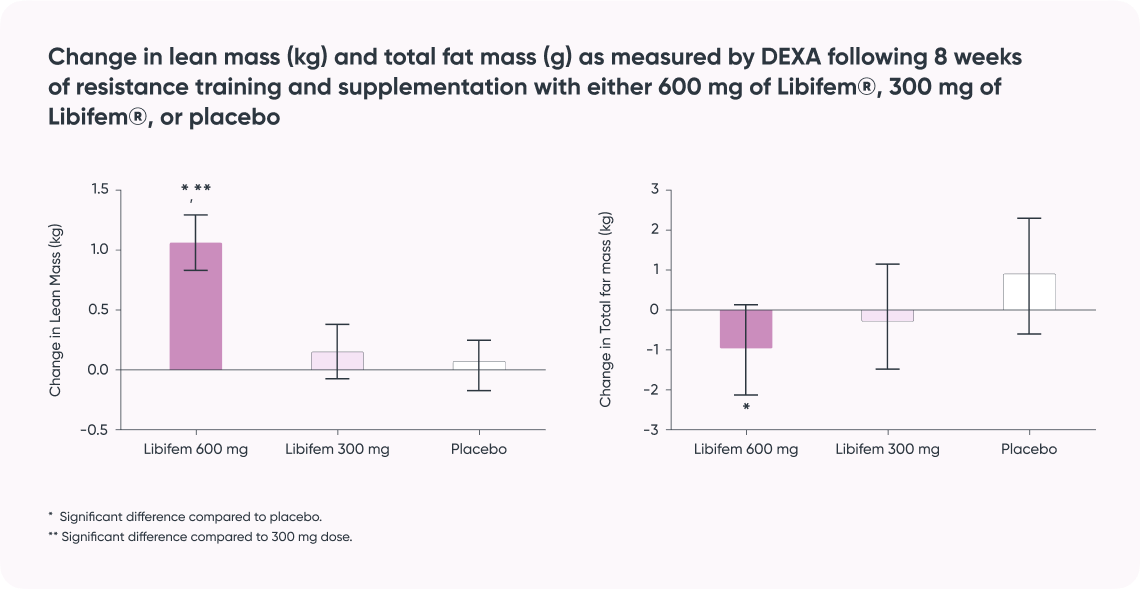
Study result:
- Supports increased strength, power, and endurance for improved athletic performance*
- Positive impact on aerobic capacity for endurance athletes*
- Supports improvement in body composition for increased metabolism and sports performance*)

